Making Allogeneic CAR T a Reality for Patients
Harnessing the spirit of cooperation needed to bring about the next cell therapy revolution… TOGETHER

What's needed. What's next.
Leaders in CAR T research provide their insights about what’s needed to deliver an off-the-shelf CAR T to patients unable to wait for a personalized treatment.
About CAR T Together™
CAR T Together™ is a first-of-its-kind effort representing the many investigators who are committed to supporting the development of “off-the-shelf” (allogeneic) CAR T products that are scalable and faster to deliver – making treatments accessible to more cancer patients.
First-generation CAR Ts (autologous) changed how many difficult-to-treat cancers are managed, but, as more patients become eligible for treatment, the arduous, individualized manufacturing process and complex supply chain may make it even harder for drugmakers to keep up with growing demand.
In fact, a new survey focused on the challenges associated with the real-world delivery of autologous CAR T therapies revealed that only half of cancer patients who are eligible for currently FDA-approved CAR T receive these treatments.
To accelerate this next revolution in CAR Ts, oncologists from preeminent research institutions nationwide have come together to inspire what is needed to move cancer treatment forward – advancement of science, supporting development of next-generation therapies and identifying and addressing the limitations of current therapies.
Equal parts collaboration, innovation and compassion, CAR T Together™ harnesses the spirit of cooperation needed to bring about the next cell therapy revolution and will collaborate with investigators and institutions in an effort to expedite clinical trial enrollment.

First-generation CAR Ts changed how many difficult-to-treat cancers are managed, but as more patients become eligible for treatment, the arduous, individualized manufacturing process and complex supply chain may make it even harder for drugmakers to keep up with growing demand.
New Survey Examines Real-World Challenges1
The global CAR T market is expected to expand dramatically as more patients become eligible in earlier stages of their disease, exacerbating an access bottleneck for patients.² Unlike first-generation CAR Ts (autologous) that are manufactured using T cells from cancer patients, allogeneic CAR Ts utilize cells from healthy donors, making them “off-the-shelf” in nature and able to be efficiently manufactured in large batches and kept frozen for on-demand delivery to patients.
While 82% of respondents agree that CAR T therapies have changed how they manage aggressive cancers, extensive wait times and manufacturing limitations keep many eligible patients from receiving the treatments.¹
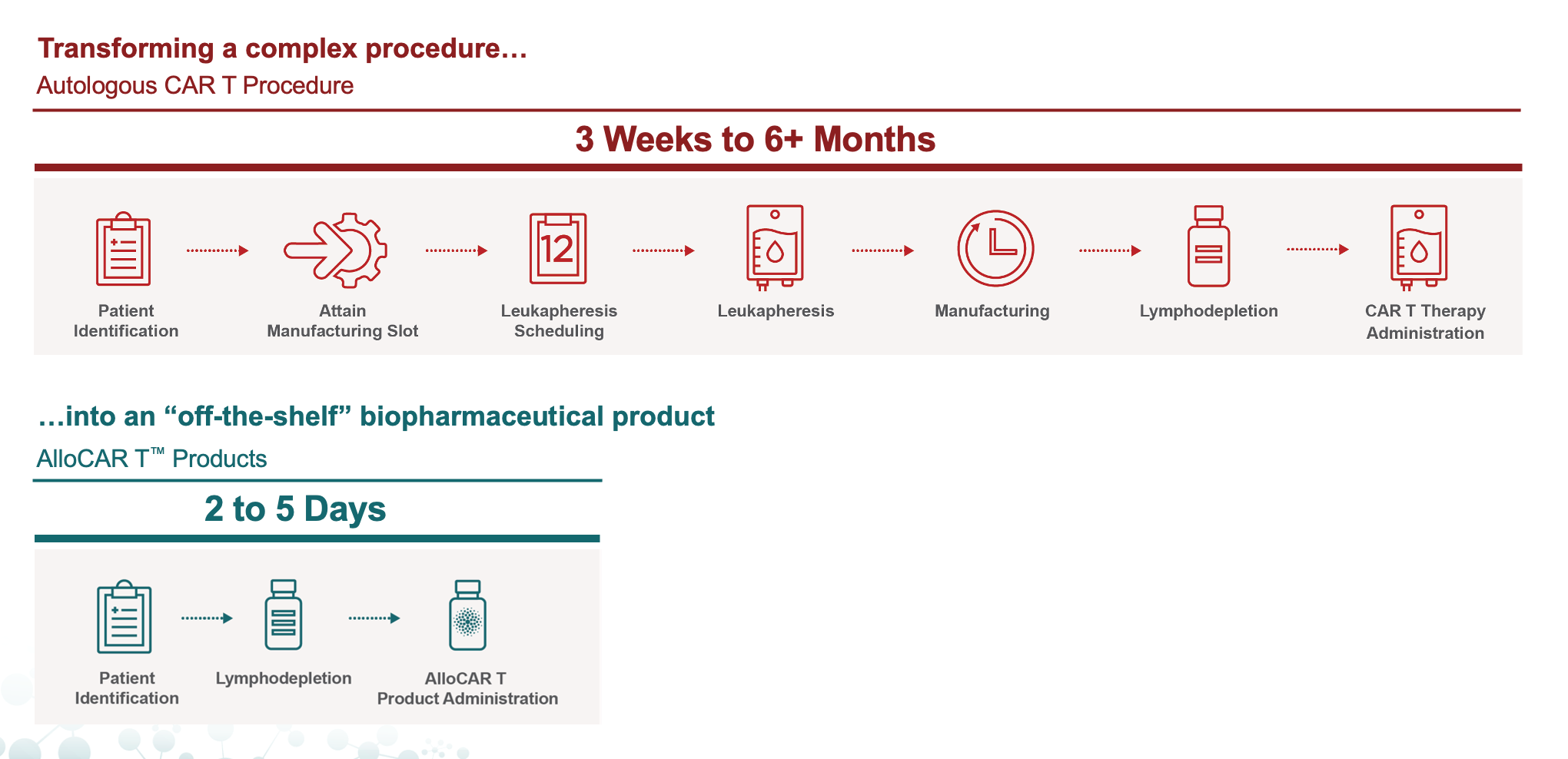
Comparing CAR T Delivery: Allogeneic Product Candidates and Autologous Therapies
A survey of 50 U.S.-based hematologist-oncologists, physician assistants, nurse practitioners, and registered nurses from academic centers with CAR T therapy capabilities is the most recent in-depth look at the challenges associated with real-world delivery of autologous CAR T therapies.
While respondents agree that CAR T therapy changed how they manage difficult-to-treat cancers, a new survey focused on access to cell therapies reveals that only half of cancer patients who are eligible for currently FDA-approved autologous CAR T therapies receive these treatments.
Of those patients eligible for treatment, 12% were able to receive treatment within one month, with approximately 40% waiting three to six months or longer to receive treatment as their disease worsens.¹ For eligible patients, disease progression, manufacturing capacity and comorbidities were top barriers.
The survey was sponsored by Allogene Therapeutics and conducted by an independent third-party research organization. The survey did not assess the treatment status of individual patients.
The global CAR T market is expected to expand dramatically as more patients become eligible², and respondents ranked increased patient demand, manufacturing capacity and time to treatment as the three biggest challenges facing CAR T adoption in the future.¹
Ushering in a New Era of Allogeneic CAR T Products

Dr. Frederick Locke
Chair, Department of Blood and Marrow Transplant and Cellular Immunotherapy; program co-leader, Immuno-Oncology, Moffitt Cancer Center
Tampa, Florida
Dr. Locke is a medical oncologist and translational researcher in the Department of Blood and Marrow Transplant and Cellular Immunotherapy. He leads the Immune Cell Therapy (ICE-T) initiative at Moffitt, an integrated cross-departmental translational team. Dr. Locke is a clinical research leader in the field of Chimeric Antigen Receptor (CAR) T cell therapy, acting as a national P.I. for several pivotal trials of anti-CD19 CARs for lymphoma. Dr. Locke provides care for multiple myeloma, lymphoma, and leukemia patients. He oversees both autologous and allogeneic hematopoietic stem cell transplants as well as cellular immunotherapy treatments such as CAR T cell Therapy.

Dr. Michael Tees
Associate member physician, director of the Lymphoma Program, Colorado Blood Cancer Institute
Denver, Colorado
Dr. Michael Tees is an Associate Member Physician at the Colorado Blood Cancer (CBCI) Institute and part of the Lymphoid and Autoimmune Disease Groups. Dr. Tees is board certified in Hematology, Medical Oncology, and Internal Medicine. His research interests focus on novel cellular and immune-based therapies in those with lymphomas and other lymphocytic disorders. He currently is a member of the American Society of Hematology, the American Society of Clinical Oncology, and the American Society of Transplantation and Cellular Therapy. In addition, he is a member of the Sarah Cannon Lymphoma Working Group.

Dr. Javier Munoz
Mayo Clinic
Phoenix, Arizona
Dr. Munoz specializes in treating patients with lymphoma and serves as the Director of the Lymphoma Program at Mayo Clinic in Arizona. He is triple board certified in Internal Medicine, Hematology and Medical Oncology. Dr. Munoz also completed a Master of Science in patient-based research and an Investigational Cancer Therapeutics fellowship at the University of Texas MD Anderson Cancer Center in Houston before relocating to Arizona to spearhead the development of Chimeric Antigen Receptors (CAR) T-cells. He has been principal investigator in multiple cutting-edge lymphoma trials including immunotherapy and CAR T-cells.

Dr. Jeffery Matous
Member physician, director of the Multiple Myeloma Program, Colorado Blood Cancer Institute
Denver, Colorado
Dr. Matous is a Member Physician at the Colorado Blood Cancer Institute (CBCI) and part of the Plasma Cell Diseases Group. He presently serves as a clinical professor of medicine at the University of Colorado Health Sciences Center and is also on the board of the Leukemia & Lymphoma Society. Dr. Matous specializes in the treatment of blood cancers such as multiple myeloma, Waldenstrom Macroglobulinemia and amyloidosis as well as in the field of blood & marrow transplantation. He is the lead investigator in clinical trials in the areas of Waldenstrom’s Macroglobulinemia, multiple myeloma, and amyloidosis, and has been recognized by his peers as a Top Doctor for the past 16 years.
Ushering in a New Era of Allogeneic CAR T Products
Dr. Frederick Locke
Chair, Department of Blood and Marrow Transplant and Cellular Immunotherapy; program co-leader, Immuno-Oncology, Moffitt Cancer Center
Tampa, Florida
Dr. Locke is a medical oncologist and translational researcher in the Department of Blood and Marrow Transplant and Cellular Immunotherapy. He leads the Immune Cell Therapy (ICE-T) initiative at Moffitt, an integrated cross-departmental translational team. Dr. Locke is a clinical research leader in the field of Chimeric Antigen Receptor (CAR) T cell therapy, acting as a national P.I. for several pivotal trials of anti-CD19 CARs for lymphoma. Dr. Locke provides care for multiple myeloma, lymphoma, and leukemia patients. He oversees both autologous and allogeneic hematopoietic stem cell transplants as well as cellular immunotherapy treatments such as CAR T cell Therapy.
Dr. Michael Tees
Associate member physician, director of the Lymphoma Program, Colorado Blood Cancer Institute
Denver, Colorado
Dr. Michael Tees is an Associate Member Physician at the Colorado Blood Cancer (CBCI) Institute and part of the Lymphoid and Autoimmune Disease Groups. Dr. Tees is board certified in Hematology, Medical Oncology, and Internal Medicine. His research interests focus on novel cellular and immune-based therapies in those with lymphomas and other lymphocytic disorders. He currently is a member of the American Society of Hematology, the American Society of Clinical Oncology, and the American Society of Transplantation and Cellular Therapy. In addition, he is a member of the Sarah Cannon Lymphoma Working Group.
Dr. Javier Munoz
Mayo Clinic
Phoenix, Arizona
Dr. Munoz specializes in treating patients with lymphoma and serves as the Director of the Lymphoma Program at Mayo Clinic in Arizona. He is triple board certified in Internal Medicine, Hematology and Medical Oncology. Dr. Munoz also completed a Master of Science in patient-based research and an Investigational Cancer Therapeutics fellowship at the University of Texas MD Anderson Cancer Center in Houston before relocating to Arizona to spearhead the development of Chimeric Antigen Receptors (CAR) T-cells. He has been principal investigator in multiple cutting-edge lymphoma trials including immunotherapy and CAR T-cells.
Dr. Jeffery Matous
Member physician, director of the Multiple Myeloma Program, Colorado Blood Cancer Institute
Denver, Colorado
Dr. Matous is a Member Physician at the Colorado Blood Cancer Institute (CBCI) and part of the Plasma Cell Diseases Group. He presently serves as a clinical professor of medicine at the University of Colorado Health Sciences Center and is also on the board of the Leukemia & Lymphoma Society. Dr. Matous specializes in the treatment of blood cancers such as multiple myeloma, Waldenstrom Macroglobulinemia and amyloidosis as well as in the field of blood & marrow transplantation. He is the lead investigator in clinical trials in the areas of Waldenstrom’s Macroglobulinemia, multiple myeloma, and amyloidosis, and has been recognized by his peers as a Top Doctor for the past 16 years.
Dr. Maung Myo Htut
Moffitt Cancer Center
Tampa, Florida
Dr. Myo Htut is a Medical Oncology Specialist in Duarte, CA and has over 27 years of experience in the medical field. He is affiliated with City Of Hope Helford Clinical Research Hospital.
He is determined to find more effective treatments for multiple myeloma, a rare blood cancer which strikes about 30,000 Americans each year. He believes the body’s own immune system may hold the key. He’s especially excited about the potential of CAR T cells – immune cells taken from the patient, re-engineered to destroy cancer, then re-introduced. Dr. Htut is conducting multiple clinical trials examining immunotherapy options for multiple myeloma.
Dr. Leslie Popplewell
City of Hope
Los Angeles, California
Dr. Popplewell is Division Chief and Associate Professor at City of Hope’s Toni Stephenson Lymphoma Center and serves as the principal investigator for a number of clinical trials examining new lymphoma therapies including CAR T cells and phase 1 and first in human trials. She is also working on advanced transplant techniques for patients whose lymphoma has recurred. Dr. Popplewell received her medical degree from the University of Louisville School of Medicine. She joined City of Hope in 1998 following a fellowship at the renowned Memorial Sloan Kettering Cancer Center in New York City.
Dr. Sumanta ('Monty') Pal
Moffitt Cancer Center
Tampa, Florida
Dr. Pal is an internationally recognized leader in the area of genitourinary cancers, including kidney, bladder, and prostate cancer. He is co-director of City of Hope’s Kidney Cancer Program and is the head of the kidney and bladder cancer disease team at the institution. Dr. Pal maintains one of the largest portfolios of clinical trials for kidney and bladder cancer research on the West Coast. He developed an integrated program that focuses heavily on collaborations with basic science researchers at Beckman Research Institute of City of Hope and urologists in the Department of Surgery.
Dr. Shahbaz Malik
Sarah Cannon Research Institute
Austin, Texas
Dr. Malik is a medical oncology and internal medicine board-certified hematologist in Austin, Texas. He specializes in cancers of the blood and bone and marrow transplants. He joined St. David’s South Austin and the Texas Transplant Institute – Center for Blood Cancers and Oncology in 2016. Dr. Malik earned his medical degree from the Medical College of Virginia and completed his residency training in internal medicine at Cleveland Clinic Foundation. He furthered his medical training, completing a fellowship in hematology/oncology at University of Maryland and a fellowship in blood and marrow transplantation at Stanford University.

Dr. Leslie Popplewell
City of Hope
Los Angeles, California
Dr. Popplewell is Division Chief and Associate Professor at City of Hope’s Toni Stephenson Lymphoma Center and serves as the principal investigator for a number of clinical trials examining new lymphoma therapies including CAR T cells and phase 1 and first in human trials. She is also working on advanced transplant techniques for patients whose lymphoma has recurred. Dr. Popplewell received her medical degree from the University of Louisville School of Medicine. She joined City of Hope in 1998 following a fellowship at the renowned Memorial Sloan Kettering Cancer Center in New York City.

Dr. Sumanta ('Monty') Pal
City of Hope
Los Angeles, California
Dr. Pal is an internationally recognized leader in the area of genitourinary cancers, including kidney, bladder, and prostate cancer. He is co-director of City of Hope’s Kidney Cancer Program and is the head of the kidney and bladder cancer disease team at the institution. Dr. Pal maintains one of the largest portfolios of clinical trials for kidney and bladder cancer research on the West Coast. He developed an integrated program that focuses heavily on collaborations with basic science researchers at Beckman Research Institute of City of Hope and urologists in the Department of Surgery.

Dr. Shahbaz Malik
Sarah Cannon Research Institute
Austin, Texas
Dr. Malik is a medical oncology and internal medicine board-certified hematologist in Austin, Texas. He specializes in cancers of the blood and bone and marrow transplants. He joined St. David’s South Austin and the Texas Transplant Institute – Center for Blood Cancers and Oncology in 2016. Dr. Malik earned his medical degree from the Medical College of Virginia and completed his residency training in internal medicine at Cleveland Clinic Foundation. He furthered his medical training, completing a fellowship in hematology/oncology at University of Maryland and a fellowship in blood and marrow transplantation at Stanford University.

Dr. Maung Myo Htut
City of Hope
Los Angeles, California
Dr. Htut is an Associate Professor in City of Hope’s Division of Multiple Myeloma and has over 27 years of experience in the medical field.
He is determined to find more effective treatments for multiple myeloma, a rare blood cancer which affects about 30,000 Americans each year. He believes the body’s own immune system may hold the key. He’s especially excited about the potential of CAR T cells – immune cells taken from the patient, re-engineered to destroy cancer, then re-introduced. Dr. Htut is conducting multiple clinical trials examining immunotherapy options for multiple myeloma.
Treatment Demand Creates a Bottleneck
First-generation CAR Ts (autologous) changed how many difficult-to-treat blood cancers are managed, giving hope for the possibility of a cure for eligible patients who have few other options. However, as more patients become eligible for treatment, the arduous, individualized manufacturing process and complex supply chain, may create an even greater access bottleneck.
Patients awaiting current autologous CAR T therapies may need to wait months to receive treatment, all the while their disease may progress. This impacted need may worsen as autologous CAR T therapies are approved in earlier lines of treatment.³
Autologous CAR Ts are manufactured by taking T cells from a patient and engineering them in a laboratory to target and attack certain cancer cells before being reinfused back into the patient. For an autologous CAR T to treat 1,000 patients, it must successfully execute 1,000 manufacturing runs.
A next-generation CAR T therapy, known as allogeneic, or “off-the-shelf,” has the potential to further revolutionize cancer care by providing broader access for patients who are critically ill.
Unlike first-generation CAR Ts (autologous) that are manufactured using T cells from cancer patients, allogeneic CAR Ts utilize cells from healthy donors, making them “off-the-shelf” in nature and able to be efficiently manufactured in large batches and kept frozen for on-demand delivery to patients. These allogeneic CAR Ts have the potential to produce enough treatments for ~100 patients in a single manufacturing run, possibly treating 20,000 patients annually from one manufacturing facility at scale.
1Allogene Data on File.
2Clarivate/Decision Resources. Data on File.
3DOI: 10.1200/JCO.2022.40.16_suppl.e20021 Journal of Clinical Oncology 40, no. 16_suppl (June 01, 2022) e20021-e20021. Published online June 02, 2022.
Real-world access to CAR T remains challenging due to supply chain limitations impacting manufacturing. One study found that the median waiting time for an FDA-approved CAR T treatment for multiple myeloma patients was 6 months with only 25% of patients receiving CAR T eventually.³